InMode Ltd, a leading global provider of innovative medical technologies, introduces Evoke, a breakthrough non-invasive solution for facial remodelling.
Evoke is the first hands-free device for the face and submental area cleared by the FDA. This advanced radiofrequency (RF) based platform addresses patient aesthetic concerns with uniform and controlled heating of the skin and subdermal tissue.
Dr Michael Kreindel, InMode Chief Technology Officer, and inventor of Evoke commented, "the idea of a facial hands-free treatment has been circulating in the market for about eight years. I'm proud that InMode, after years of research and development, is the first company to bring this solution to patients worldwide."
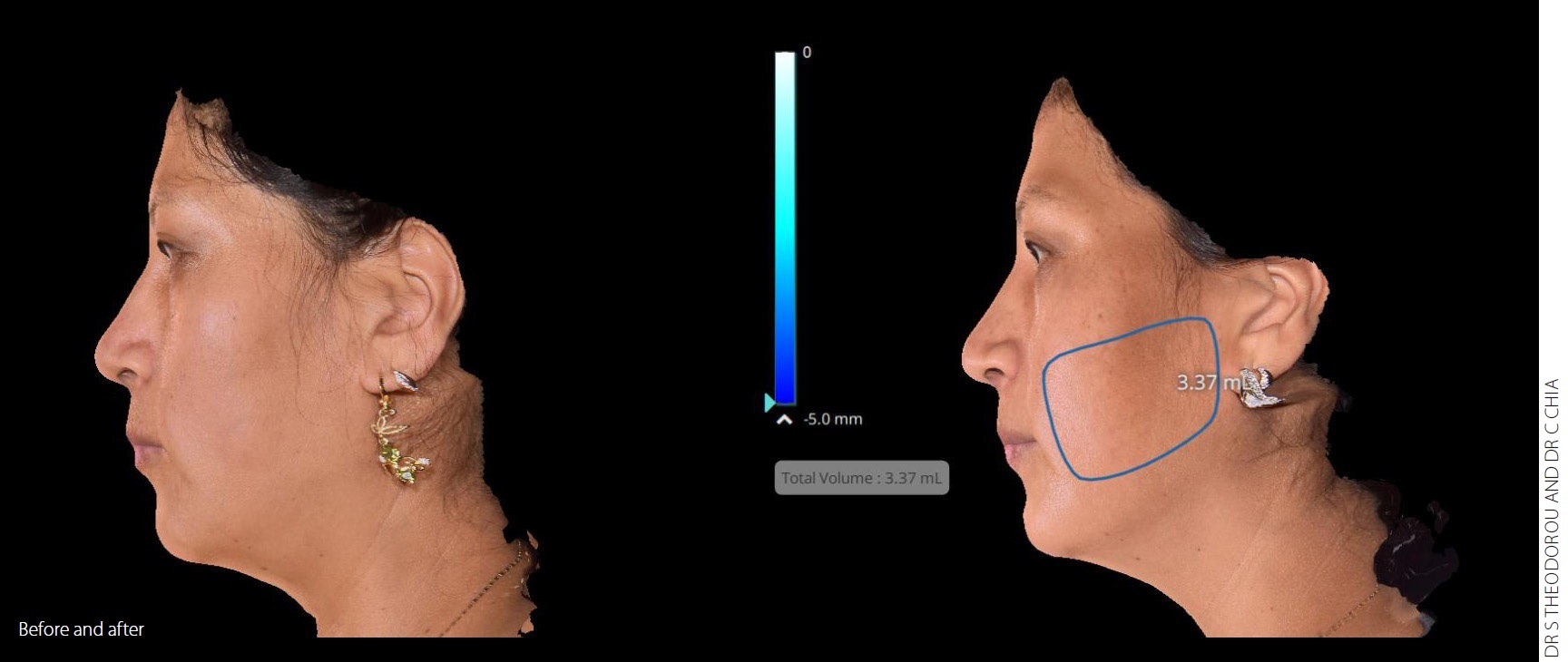
"Evoke delivers the ultimate solution in thermal facial procedures. Patients can not only turn back the hands of time but are now also able to achieve long-lasting structural enhancements to their face without excisional surgery. InMode once again has brought to market another aesthetics industry game changer," said Dr Spero Theodorou, InMode Chief Medical Officer and Plastic Surgeon (bodySCULPT, NY).
About InMode
InMode is a leading global provider of innovative medical technologies. InMode develops, manufactures, and markets devices harnessing novel radiofrequency technology. InMode strives to enable new emerging surgical procedures as well as improve existing treatments. InMode has leveraged its medically-accepted minimally-invasive RF technologies to offer a comprehensive line of products across several categories for plastic surgery, gynaecology, dermatology, otolaryngology, and ophthalmology.
For more information about InMode and its wide array of medical technologies, visit www.inmodemd.co.uk

 Added to basket
Added to basket

 Unapplied Changes
Unapplied Changes