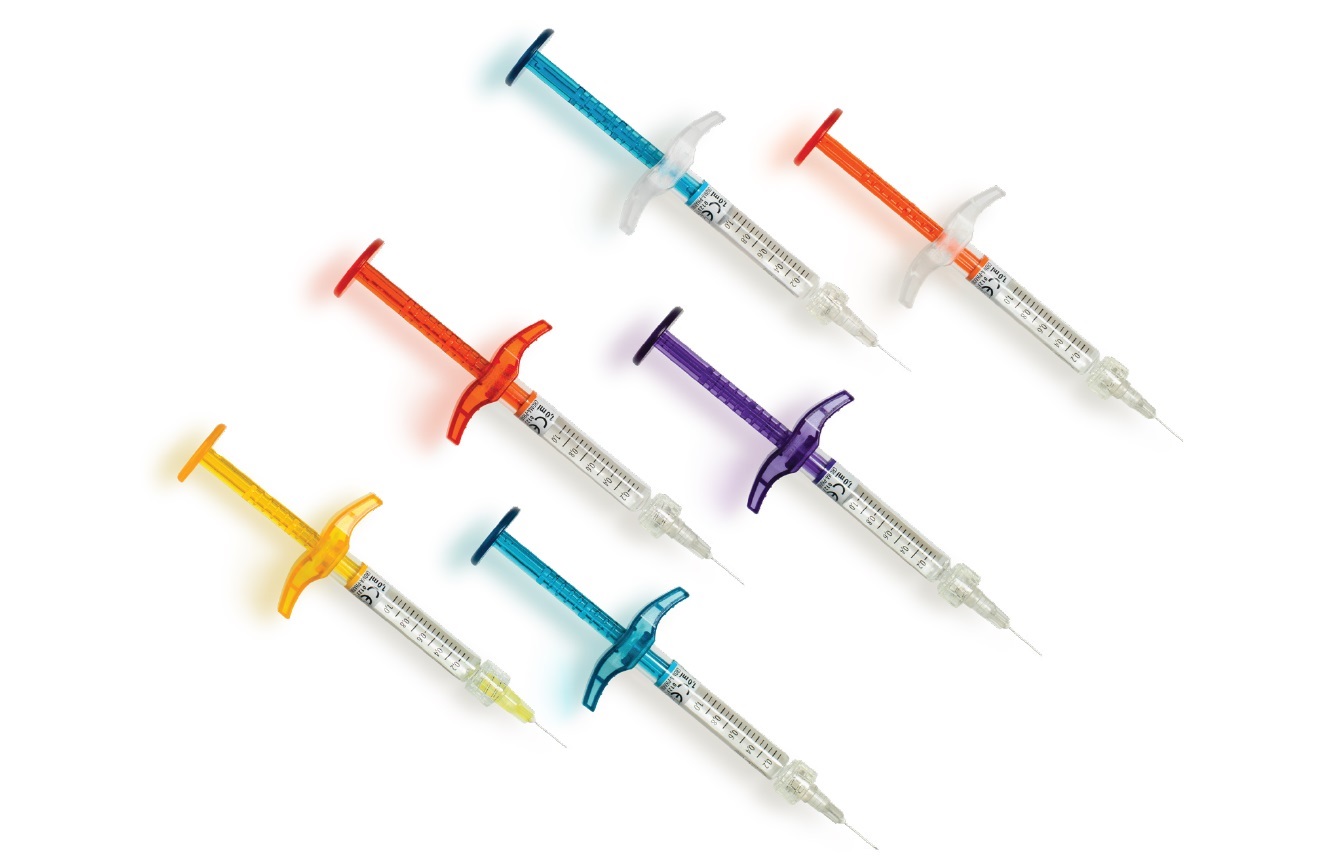
The saypha® rebrand introduces NEW ComfortFlow colour coded syringes for clear product differentiation.
The premium glass syringe design has been developed and tested together with healthcare professionals to optimise comfort and ease whilst injecting.
At the core of Croma’s comprehensive aesthetic portfolio, the saypha® HA filler range is produced in one of the most modern manufacturing sites in the world in Austria.
With over 100 million syringes produced and 80.000 syringes per batch, Croma proudly provides a safe and effective portfolio to Health Care Professionals in more than 70 countries worldwide.
Pioneers in HA manufacturing Heritage & Innovation
A modern and automated production plant in Austria, with minimal batch to batch variation and human error. Croma’s proprietary MACRO Core Technology enables manufacturing of saypha® with predefined viscoelastic characteristics and contributes to product purity.
Proven Efficacy & Reliability
In seven clinical trials involving 470 patients, Croma showed that the portfolio ensures the highest standards for use in the aesthetic market.Responder rate exceeded 95%.1,4-792% of patients sustained aesthetic improvement until month 13.2,1
Patient Satisfaction & Safety.
90% of the patients were satisfied with the treatment results at month 9.3, 4-7Continuous monitoring of products placed. 8-10
MDR approved saypha®
Available in the UK & Ireland with clinically proven safety, effectiveness, new branding and design.
New EU MDR regulations are being put into place to improve the safety and performance of medical devices for aesthetic purposes in Europe in order to provide a high level of protection for the health of patients and users of medical devices.
Having obtained worldwide first MDR approval (Annex XVI) for saypha® RICH in October 2023 for aesthetic purposes, Croma announced MDR approval for the full saypha® HA filler portfolio at AMWC 2024 in Monaco. With this milestone, Croma-Pharma guarantees a safe and efficacious product portfolio produced under the highest standards for their distribution partners, HCPs and end users.
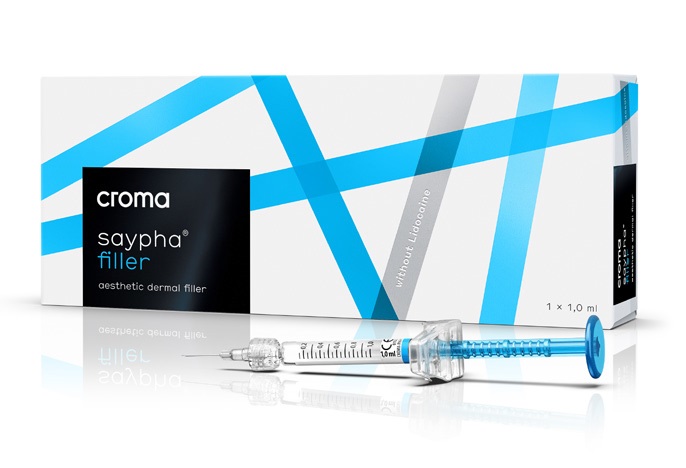
saypha® FILLER
To correct moderate to severe nasolabial folds. For cosmetic and medical reconstructive purposes in the treatment of facial lipoatrophy, debilitating scars or morphological asymmetry.
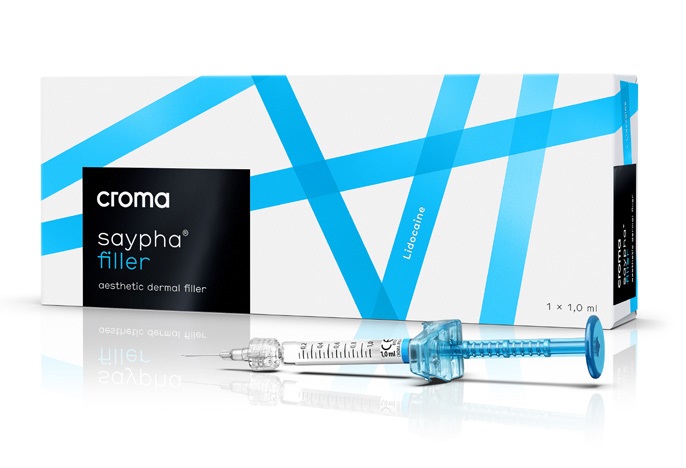 #
#saypha® FILLER Lidocaine
To create volume in order to correct wrinkles and folds in order to treat signs of ageing.
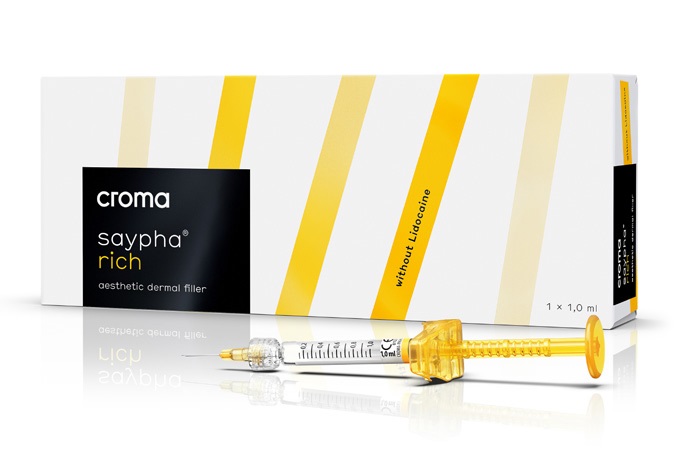
saypha® RICH
A viscoelastic solution that restores hyaluronic acid loss, maintaining skin hydration, improving tone and elasticity, and acting as a filler for small lines like crow’s feet and smile lines.
saypha® VOLUME
For correcting moderate to severe nasolabial folds and medical reconstructive treatment, for instance, of facial lipoatrophy, debilitating scars, or morphological asymmetry
saypha® VOLUME Lidocaine
To create volume in order to correct wrinkles and folds in order to treat signs of ageing.
saypha® VOLUME PLUS
The intended purpose of the device is to restore facial volume in order to correct moderate to severe midface volume deficit to treat signs of ageing.

Croma-Pharma® is a global player in the minimally invasive aesthetics market and a leading European manufacturer of premium quality hyaluronic acid syringes. The company offers a comprehensive and innovative aesthetics portfolio including botulinum toxin, fillers, lifting threads, Polynucleotides, PRP and biostimulators complemented by its own skincare brand. Founded in 1976 by the pharmacist couple Prinz, Croma-Pharma® GmbH is a family company headquartered in Austria where it also operates its manufacturing plant. With 550 employees, 13 subsidiaries in Europe and Brazil, two joint ventures and 60 exclusive export partners, it distributes its products in 80 markets globally, including the US/Canada, China and Australia/New Zealand. It also operates as a contract manufacturer in orthopaedics and ophthalmology. For more information please visit cromapharma.com.
References: 1. MDR (Medical Device Regulation) Annex XVI. 2. At week 4 evaluated by the investigator, ≥1 grade improvement based on different scales. 3. After initial treatment with Saypha VOLUME PLUS Lidocaine assessed by investigators based on Global Aesthetic Improvement Scale (GAIS). 4. Rzany B, Sulovsky M, Sattler G, Cecerle M, Grablowitz D. Long-term Performance and Safety of Princess Volume Plus Lidocaine for Midface Augmentation: The PRIMAvera Clinical Study. Aesthet Surg J. 2023 Jul 13:sjad230. doi: 10.1093/asj/sjad230. Epub ahead of print. PMID: 37439274. 5. Kopera D, Ivezic-Schoenfeld Z, Chang-Rodriguez S, Hoeller S, Grablowitz D, Bartsch K, Prinz M. A prospective, open label, multicenter, postmarket study evaluating Princess VOLUME Lidocaine for the correction of nasolabial folds. Dermatol Ther. 2020 Nov;33(6):e14310. doi: 10.1111/dth.14310. Epub 2020 Oct 5. PMID: 32946162. 6. Kopera D, Palatin M, Bartsch R, Bartsch K, O‘Rourke M, Höller S, Baumgartner RR, Prinz M. An open-label uncontrolled, multicenter study for the evaluation of the efficacy and safety of the dermal filler Princess VOLUME in the treatment of nasolabial folds. Biomed Res Int. 2015;2015:195328. doi: 10.1155/2015/195328 Epub 2015 Mar 3. PMID: 25821787; PMCID: PMC4363551. 7. Grablowitz D, Sulovsky M, Höller S, Ivezic-Schoenfeld Z, Chang-Rodriguez S, Prinz M. Safety And Efficacy Of Princess® FILLER Lidocaine In The Correction Of Nasolabial Folds. Clin Cosmet Investig Dermatol. 2019 Nov 26;12:857-864. doi: 10.2147/CCID.S211544. PMID: 31819583; PMCID: PMC6885652. PRSMDR0424. 8. Customer Complaints for HA fillers, Croma received as Manufacturer of Medical Devices, 2022. 9. Croma data on file: REC-0008. 10. Croma data on file: complaint rate saypha® 2022. |
For more information on the new saypha® and new MDR approvals, please contact us: info.uk@cromapharma.com

 Added to basket
Added to basket

 Unapplied Changes
Unapplied Changes